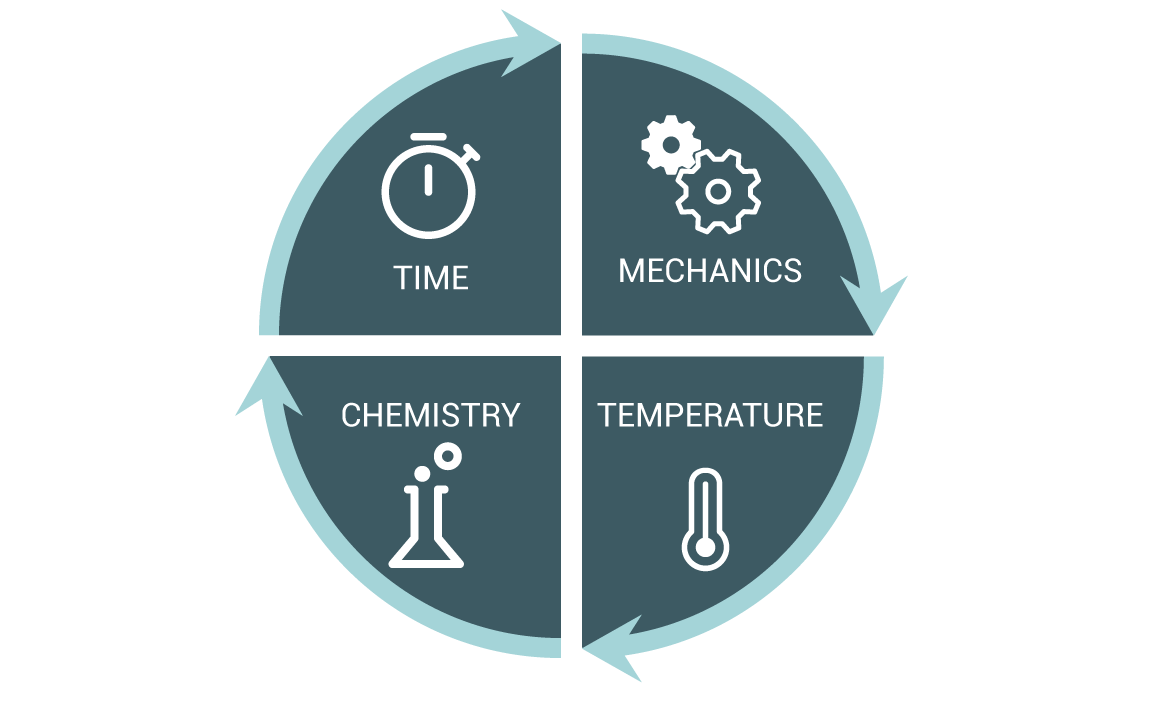
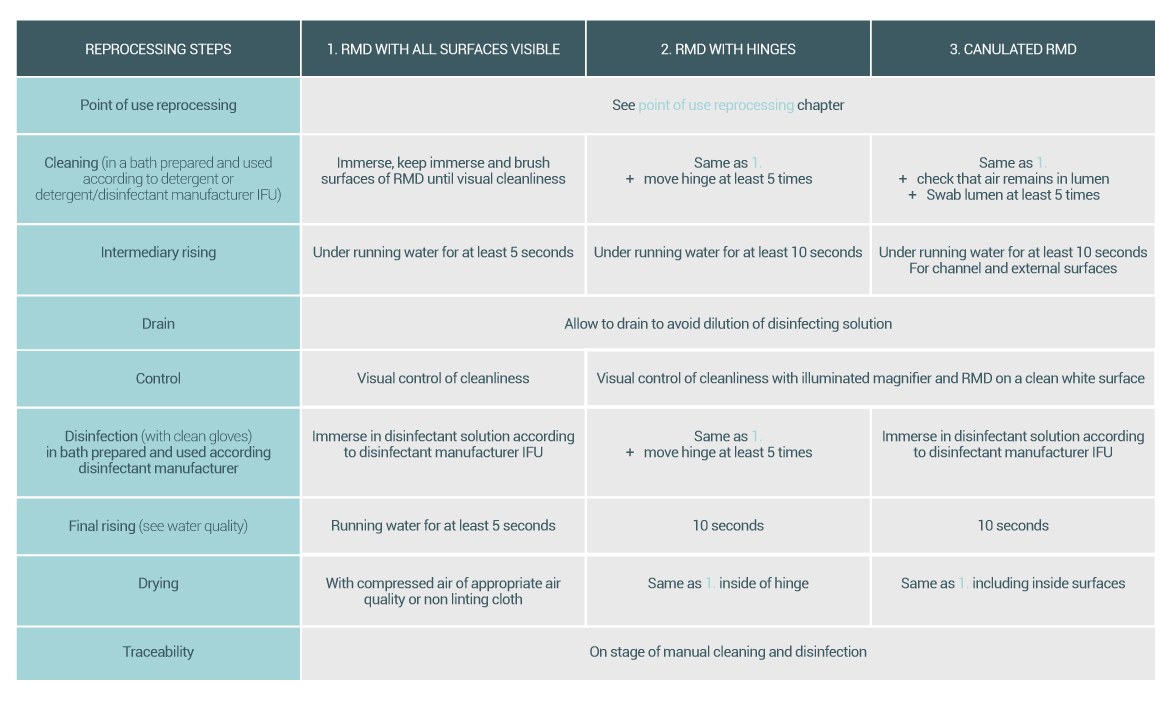
Cleaning is intended to remove soils (e.g., patient secretion, tissues and inorganic material such as salts) from surfaces of reusable medical devices (RMD). Cleaning also helps remove some of the microbial load and limits the amount of endotoxin or pyrogen residue that may remain after sterilization.
Disinfection reduces the microbial burden.
- When disinfection is thelast step before use of an RMD, its objective is to render the RMD safe for the patient according to Spaulding classification
- When disinfection is performed in preparation for sterilization (for example in automated washer-disinfectors), disinfection improves the preparation of a RMD and may be required or recommended in some countries as an occupational health and safety measure for operators in charge of packaging.
This chapter will first serve as a reminder of the objectives and principles of cleaning and disinfection before describing the various types of cleaning and disinfection processes (manual, automated, and ultrasonic cleaning) and their implementation. See flexible endoscopy for the reprocessing of thermolabile endoscopes.