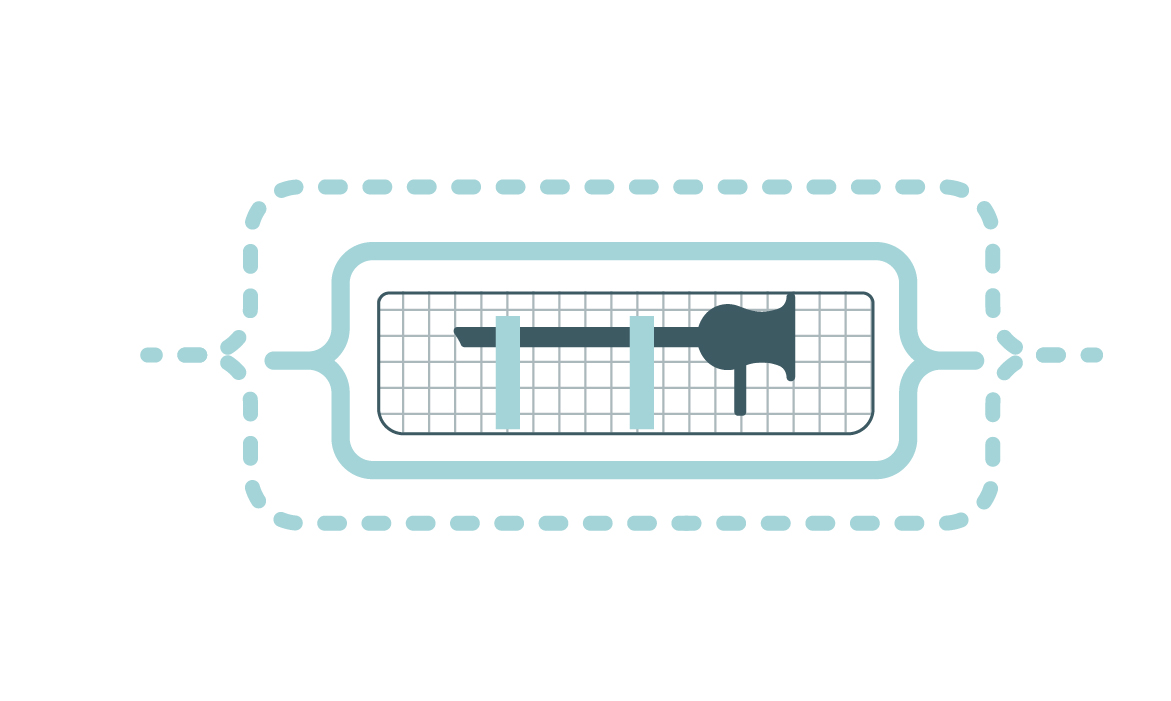
Packaging is intended to preserves the sterility of the reusable medical device (RMD) after sterilization until its use.
International standard ISO 11607-1 defines packaging functions as follows :
- The Sterile Barrier System (SBS) is “the minimum package that minimizes the ingress of microorganism and allows aseptic presentation of the sterile contents at the point of use”.
- The protective packaging (PP) is “the configuration of materials designed to prevent damage to the sterile barrier system and its contents from the time of their assembly until the point of use”.
- The packaging system (PS) is “the combination of a sterile barrier system and protective packaging”.
The sterile barrier system (SBS):
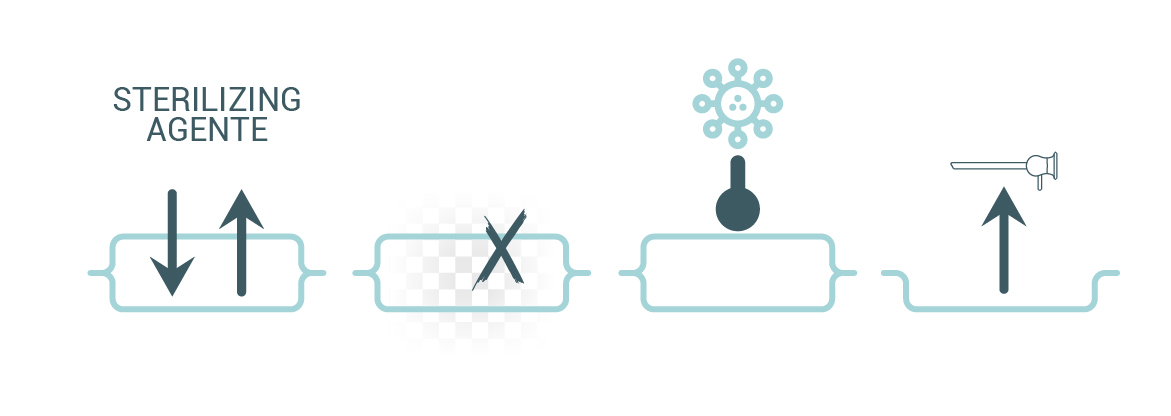
- allows the free circulation of the sterilizing agent and extraction of condensate or chemical residues and resists to high temperature (when applicable) and pressure variations,
- does not produce any toxic by-products or particles,
- protects its contents from contamination (microorganisms and dust) until use,
- allows for the aseptic presentation of the medical device.
- The packaging system is adapted to the size, weight, shape and configuration of the RMD or RMD composition.
- The packaging system ensures the physical protection the RMD and SBS during handling, transport and storage. If needed, RMDs are installed in a tray. Adapted accessories or instrument organizers protect the device and avoid breach of the SBS by sharp edges.
- As needed, the SBS is inserted into protective packaging. If the protective packaging is installed before sterilization, it must be compatible with the sterilizing agent. Contaminated protective packaging is removed before entry in the sterile area.
- If the SBS must be sterile to allow extraction of the RMD by sterilization personnel, the protective packaging is also a sterile barrier system (SBS).