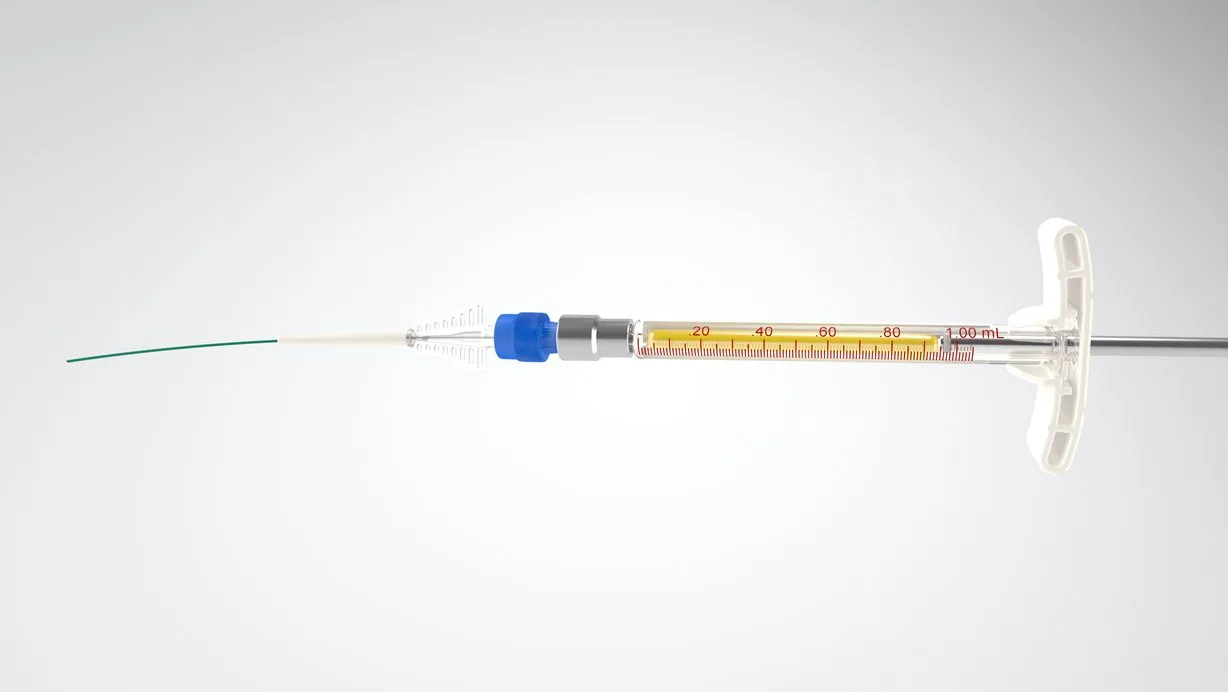
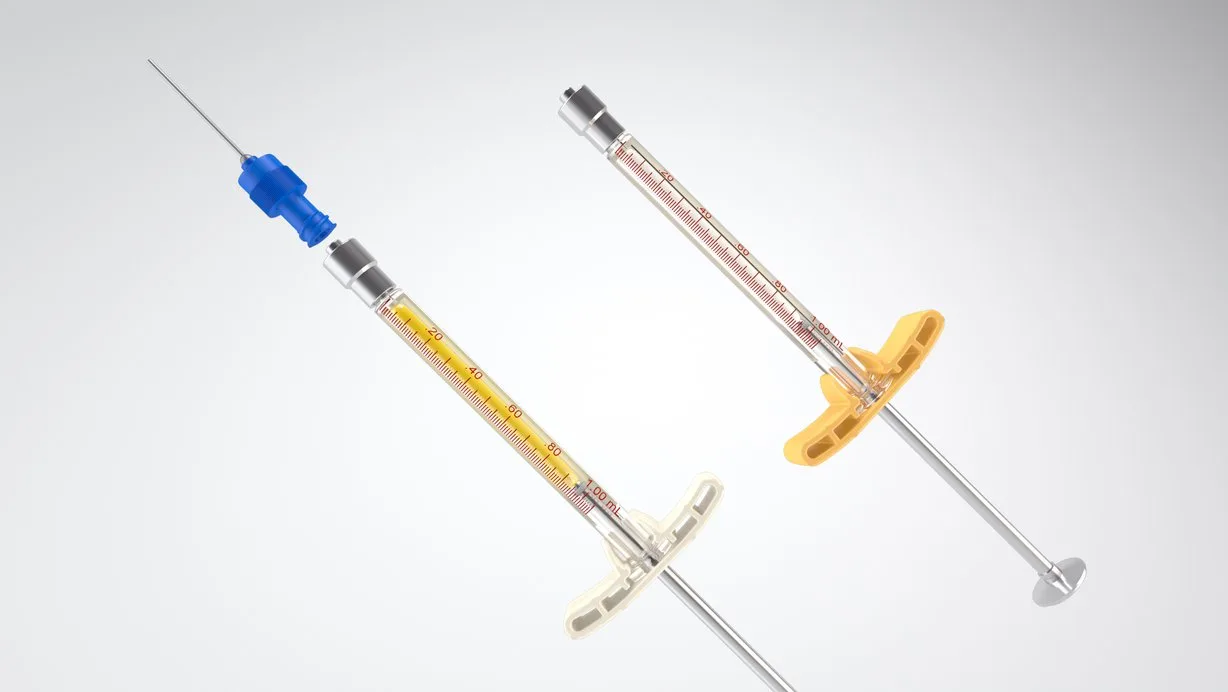
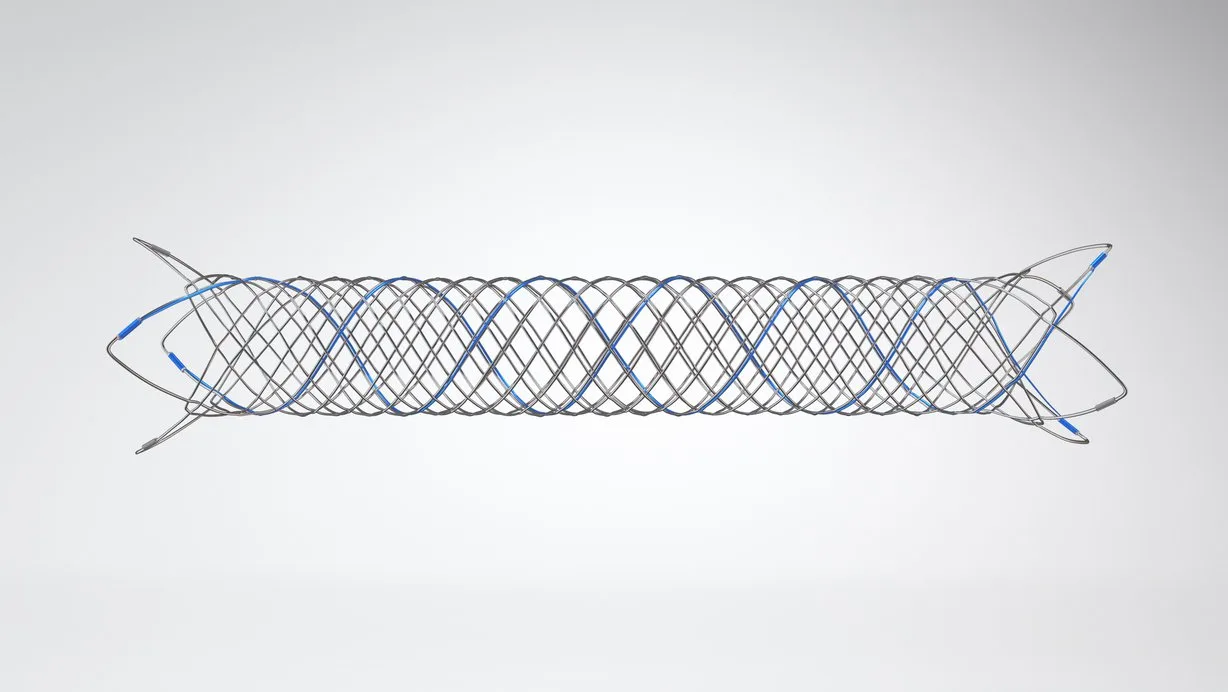
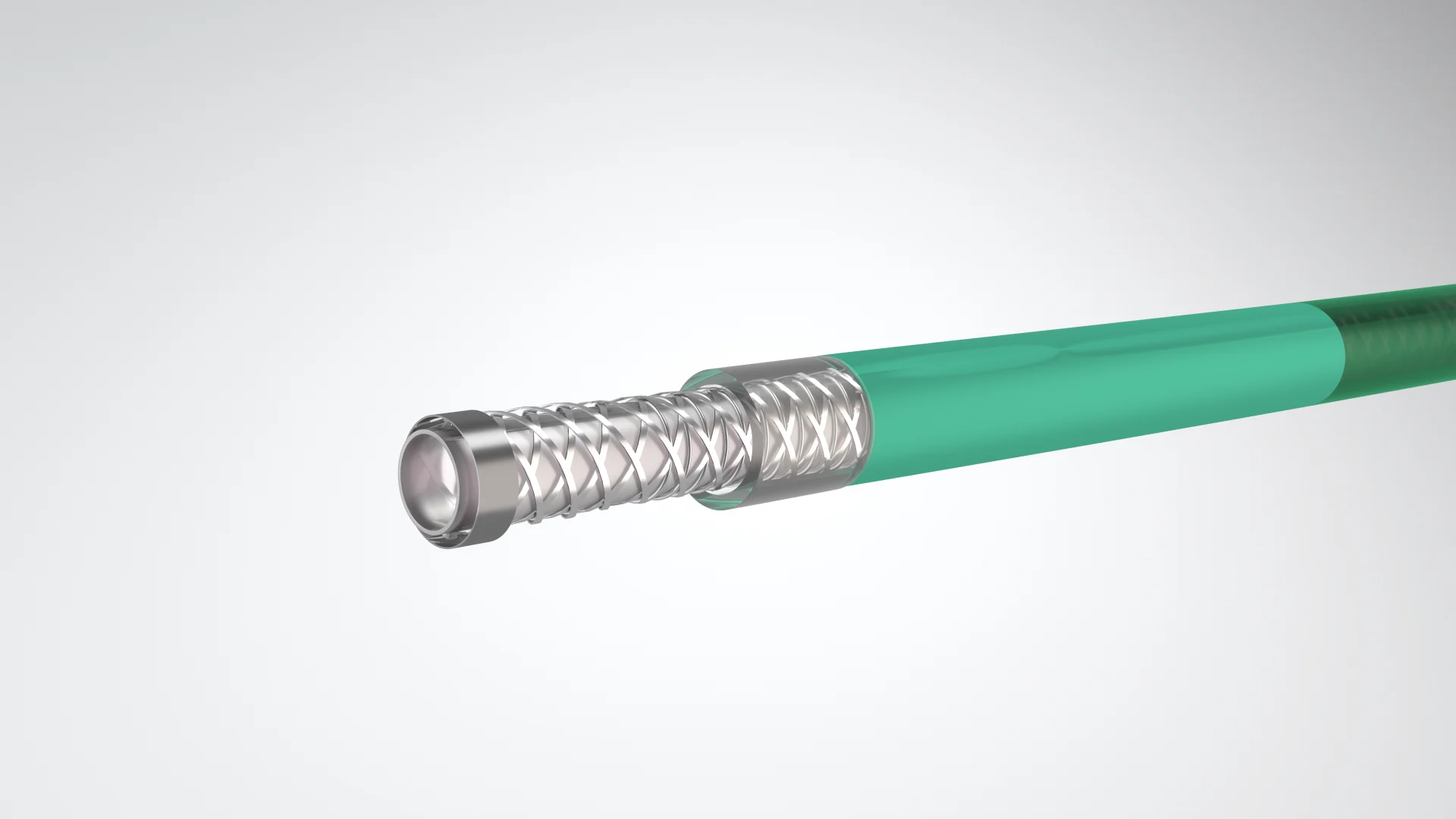
WE OPENLY DISCLOSE OUTCOMES FROM OUR CLINICAL TRIALS, REGARDLESS OF WHETHER THEY ARE NEUTRAL, NEGATIVE, OR POSITIVE. ADDITIONALLY, WE PROVIDE TRIAL VOLUNTEERS, RESEARCHERS, AND OTHERS WITH THE DATA COLLECTED IN THE CLINICAL TRIALS WE SUPPORT.
WE HOLD THE BELIEF THAT RESEARCHERS, TRIAL PARTICIPANTS, REGULATORS, AND OTHER STAKEHOLDERS WORKING IN THE BEST INTEREST OF PATIENTS SHOULD HAVE ACCESS TO CLINICAL TRIAL INFORMATION FOR THE ADVANCEMENT OF MEDICAL KNOWLEDGE AND PROGRESS. EQUALLY IMPORTANT IS ENSURING THAT THIS ACCESS IS DESIGNED TO SAFEGUARD PATIENT PRIVACY, UPHOLD REGULATORY AUTHORITY, AND MAINTAIN INCENTIVES FOR DATA GENERATORS TO ENGAGE IN FURTHER .
LET’S GET
STARTED

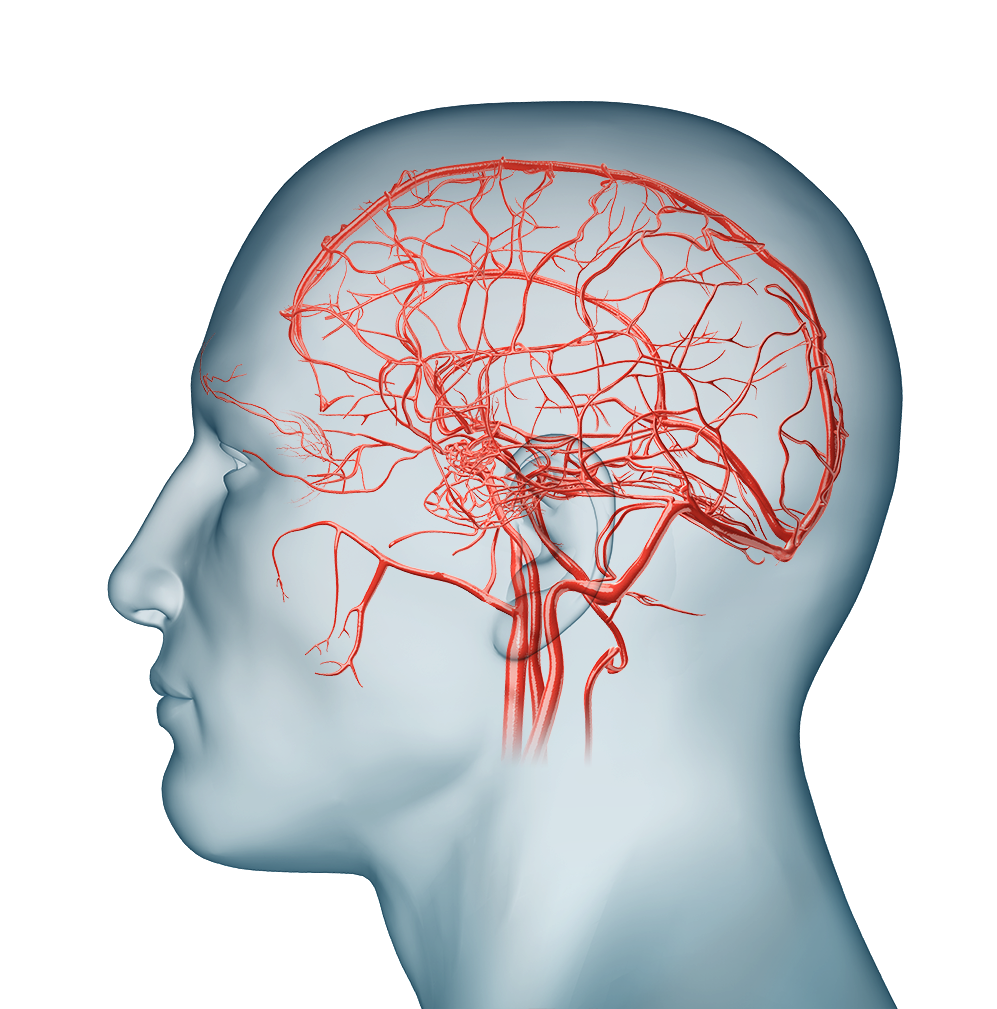

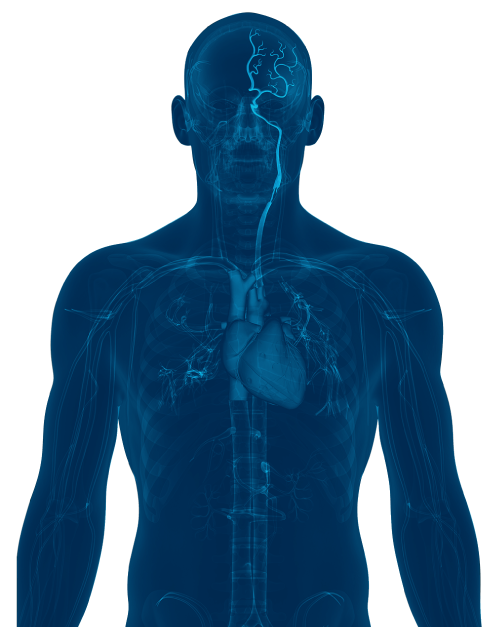





 71
71